AI-Guided CAR Design and AKT3 Degradation Synergize to Enhance Bispecific and Trispecific CAR-T Cell Persistence and Overcome Antigen Escape
Team Celogen | June 16, 2025
The structural design of chimeric antigen receptors (CARs) is critical for achieving robust and durable anti-tumor responses, particularly when targeting multiple antigens to prevent tumor antigen escape. However, increasing CAR complexity can introduce structural vulnerabilities, leading to antigen-independent T cell activation, activation-induced cell death, and reduced CAR-T cell persistence. To overcome these challenges, we designed 10,824 CAR molecules across diverse formats and screened 1,452 constructs in-vitro to develop an artificial intelligence model, termed CAR-Mediated Self-Destruction (CARMSeD), which predicts CAR designs susceptible to self-activation and dysfunction. Guided by CARMSeD and structural CAR-CAR interaction modeling, we identified optimized CAR architectures incorporating ICOS and 4-1BB co-stimulatory domains. Humanized bispecific CARs targeting CD20/CD19 and CD22/CD19 demonstrated superior anti-tumor efficacy and persistence both in-vitro and in various xenograft mice models.
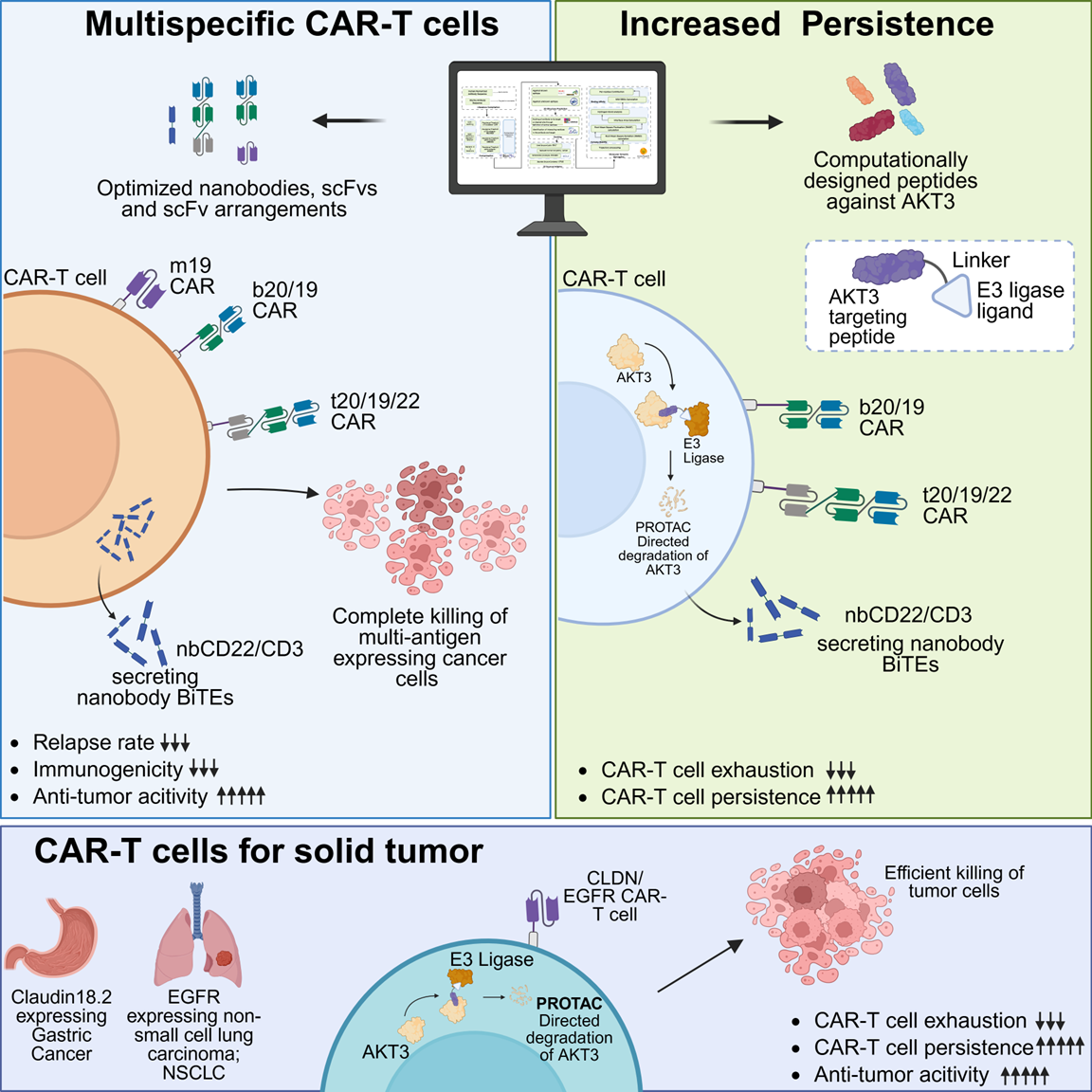
To further extend CAR-T cell persistence, we engineered bispecific CARs integrated with an AKT3-targeted PROTAC strategy. Targeted degradation of AKT3 enhanced anti-tumor potency, promoted memory T cell formation, and enabled sustained responses even under tumor rechallenge and CD19 antigen-loss conditions. Mechanistically, these effects were mediated by metabolic reprogramming involving FOXO4; notably, FOXO4-deficient CAR-T cells exhibited impaired long-term persistence. Leveraging these mechanistic insights, we developed a trispecific CAR-T cell platform incorporating a bispecific T cell engager (BiTE) targeting CD22/CD3, combined with AKT3 PROTACs. These trispecific CAR-T cells achieved potent tumor eradication, even against malignancies lacking both CD19 and CD20 expression. Collectively, this study presents a comprehensive strategy combining structure-based design, AI-guided screening, and targeted protein degradation to engineer next-generation bi and trispecific CAR-T cells with enhanced persistence, broad antigen coverage, and superior therapeutic durability.
For full article, please click the link: Click Here
Related Blogs
How we can treat Sickle Cell Disease, ....
Sickle cell disease (SCD) and thalassemia are among the ..
CAR T-Cell Therapy: A New Frontier in Treating....
Chimeric Antigen Receptor (CAR) T-cell therapy has ..

CRISPR-Cas is revolutionising Blood...
Blood cancers, including leukemia, lymphoma, and...
Indigenous CRISPR-Cas: Paving the Way for...
Cancer remains a significant public health challenge in Indi...